Stop Wasting Water and Money. FIND A LOCAL PRO
What is Mercury?

Mercury is a shiny, silver-white metallic element that exists as a liquid at room temperature, according to the MIT School of Engineering. Historically, early scientists called mercury “quicksilver.”
Mercury deposits are concentrated in the Earth’s crust and coal deposits. Naturally-occurring mercury exists as organic compounds like methylmercury. Methylmercury is the most common organic mercury compound, and according to the Environmental Protection Agency (EPA), it is highly toxic.
The EPA notes that mercury can also exist as inorganic salt compounds — mercuric sulfide, otherwise known as cinnabar, is better known than others. Mercury can combine with sulfur, chlorine, and other elements to form these inorganic salts.
Uses of Mercury
Mercury is perhaps best known as the liquid used inside some thermometers. The EPA notes that 13 states have passed laws banning the production or sale of mercury thermometers outright. The National Institute of Standards and Technology (NIST) announced they would no longer perform the initial calibration of mercury thermometers in 2011, effectively killing them for commercial use, per a write-up in Slate.
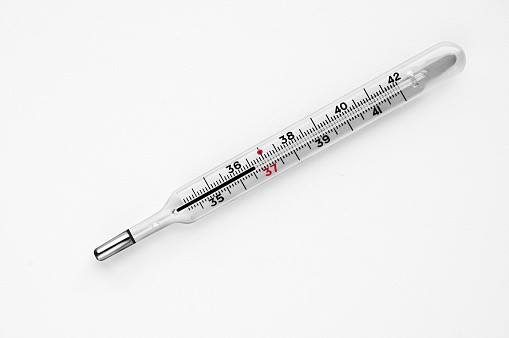
In its vapor form, mercury exists inside Compact Fluorescent Light Bulbs (CFLs). The EPA states that these bulbs use less energy and reduce greenhouse gas emissions.
Mercury is present in certain types of batteries, but its usage in the United States has declined sharply. According to the EPA, in the U.S., only button cell batteries and mercuric oxide batteries contain mercury.
Per the EPA, trace amounts of mercury exist in consumer products like appliances, jewelry, medical equipment, and automotive parts.
All forms of mercury are toxic, whether elemental mercury, mercury vapor, or methylmercury concentrated in seafood, according to the EPA. Specific protocols are in place when a product like a thermometer or a light bulb containing mercury breaks. Per the EPA, the federal government has passed many environmental laws to limit the public’s exposure to mercury.
Here’s a little-known fact for your next trivia game: the symbol of the planet Mercury represented the metal in 6th-century alchemy, and that’s where the name originated, according to the Encyclopedia Britannica. Mercury was used for medical purposes as early as 1500 BCE. In fact, the Chinese believed that mercury promoted better health and prolonged life. The first emperor of China, Qin Shi Huang, died by consuming a mixture of mercury and jade, according to the New World Encyclopedia; a concoction was created by scientists within his court who believed they were granting their emperor eternal life.
We are water Experts
Water quality is non-negotiable. To prove it, we've built state-of-the-art water quality labs and filled them with the best and brightest scientists — all so we can provide you with the best water of your life.
Sources of Mercury in Water
The EPA breaks down exactly how mercury can emit into the air, and, from there, make its way into the soil and groundwater of an area.
Several processes can create mercury emissions. Volcanoes and forest fires naturally release mercury vapors into the atmosphere. Many mercury emissions originate from man-made activities. The burning of coal, wood, and oil releases a substantial amount of mercury vapor. Certain mining practices also generate mercury vapor.
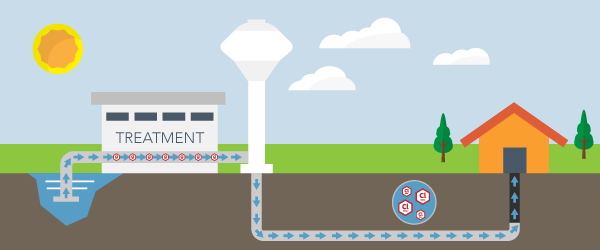
Once mercury pollution releases into the air, the vapors will fall to Earth as rain or due to gravity, a process known as air disposition. The mercury vapor can fall into freshwater bodies or fall on land where it can wash into waterways.
According to a guide from the North Carolina Department of Health and Human Services, mercury can find its way into many water supplies through this multi-step process. Mercury is present in most bodies of water, and some seafood can contain a significant amount of mercury.
Places where mercury concentrations can increase include areas near mineral deposits, mines, power plants, and industrial sites where coal burns.
Effects of Mercury on Plants and Animals

Watering plants with water containing mercury does not promote optimal plant growth. According to a scientific article published in The Botanical Review, mercury exposure affects all developmental and growth stages of plants by inhibiting photosynthesis, limiting water absorption, causing abnormal germination, and reducing biomass production.

Mercury exposure is also toxic to wildlife. Birds and other mammals eat insects and fish exposed to mercury, and as the mercury travels up the food chain, it becomes more concentrated. This process, which the National Wildlife Federation calls biomagnification, puts wildlife at risk of consuming toxic levels of mercury that can cause health issues.
How to Test Your Water for Mercury
If you are concerned about the possibility of mercury in your drinking water, multiple testing options are available. You can contact your city or county health department to request a free test for your drinking water.
After reading your water quality analysis report, it’s best to contact the lab that conducted the testing if you have any questions.
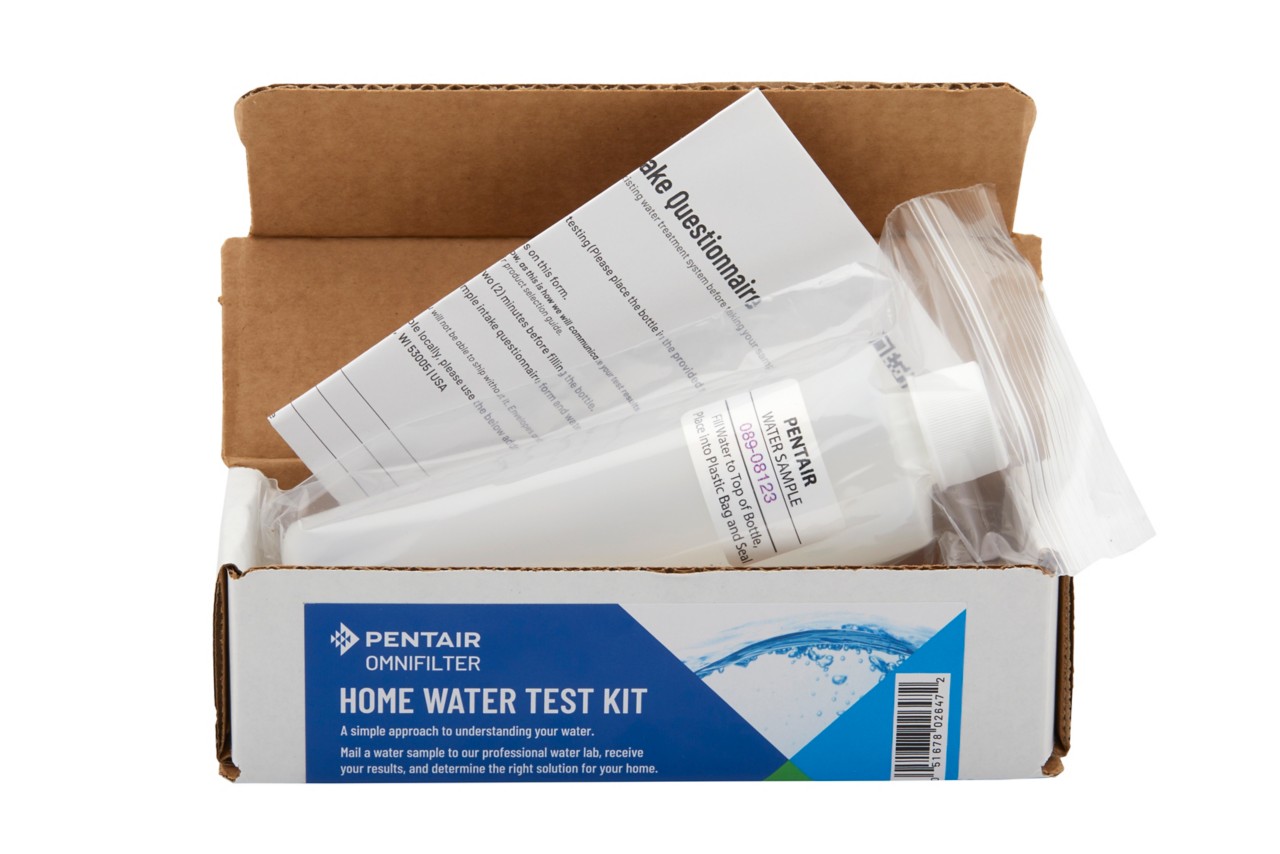
What's in your water?
start here
Worried about your water? Take control with our at-home water test kit. We'll analyze your water and recommend the best filtration or softening solution for your specific needs.
How to Reduce Mercury in Drinking Water
The EPA set the maximum contaminant level for mercury in your drinking water at 2 parts per billion (ppb), or 2 micrograms per liter. The agency recommends that homeowners take action if the mercury concentration in their water exceeds or is at that level.
Are you wondering how to reduce mercury from your water? The WQA recommends distillation and reverse osmosis to reduce mercury in your drinking water effectively.

Water 101
Unleash the secrets of your H2O! Explore where water originates and discover the various water filtration solutions that transform it into drinkable water.



